Very Short Answer Type Questions:
1 ) What is torr?
Answer : Pressure exerted by 1 mm of mercury column at 00 C and at standard gravity is called as 1 torr.
2 ) Which state of matter has no definite volume and no definite shape ?
Answer : Gaseous state has no definite volume and no definite shape.
3 ) What is the value of gas constant in SI units ?
Answer : 8.314 J/Mole/ K
4 ) How is the unit ‘L’ related to dm3?
Answer : 1 L = 1 dm3
5 ) Name the SI unit of pressure ?
Answer : Pascal
6 ) What is the value of temperature absolute zero in Celsius scale ?
Answer : -273 0C
7 ) What is meant by STP ?
Answer : STP means Standard Temperature and Pressure. The value of Standard Temperature is 0 degree centigrade and the value of standard pressure is 1 atm.
8 ) Name the law that governs the expansion of ideal gases at constant pressure.
Answer : Charles’ Law
9 ) Under what conditions is Boyle’s law is applicable ?
Answer : Under constant temperature and number of moles for given mass of a gas, Boyle’s law is applicable.
10 ) What is the effect of increase of temperature on vapor of a liquid at constant pressure ?
Ans : With the increase of temperature, vapor pressure of a liquied at constant pressure also increases.
11 ) Write down the value of R in l- atm /mole/k.
Ans : 0.0821 L – atm/ mole/ K
12 ) Express Boyle’s law and Charles’ law in the form of equation ?
Answer : Boyle’s Law in the form of equation : PV = K
Charles’ law in the form of equation : V = KT
where K is proportionality constant.
13 ) Out of dry air and wet air which is heavier ?
Answer : Wet air
14 ) What is the cause of a gas pressure ?
Answer : The hitting of the particles of the gas on the wall of the container due to Brownian motion is the cause of gas pressure.
15 ) Expandability of a gaseous substance is very high -why ?
Answer : Due to weak interaction between the gaseous molecules
16 ) What are the variables and constant quantities in Gay- Lussac’s law ?
Answer : The variable quantities are pressure and temperature while constant quantity is volume of the gas.
17) What are the constant of Boyle’s law ?
Answer : Temperature, mass and number of moles are constant of Boyle’s Law.
Short Answer Type Questions :
18 ) What is normal temperature and pressure ?
Answer : Normal temperature = 20 degree centigrade
Normal Pressure = 1 atm
19 ) Why is Boyle’s law not applied while a balloon is blown with air ?
Answer : In Boyle’s law, temperature and mass should be kept constant. With every blow, there is increment of mass of the air inside the balloon. So while a balloon is blown with air, the mass is not constant. Therefore, Boyle’s Law is not applicable in this case.
20 ) State Boyle’s law.
Answer : It states that volume of a given mass of a gas is inversely proportional to its pressure while temperature remains constant.
21 ) State Charles’ law.
Answer : It states that the volume of a givne mass is increased or decreased by 1 / 273rd parts of the volume at zero degree centigrade for every rise or fall of temperature while pressure remains constant.
22 ) Write down the relation between Celsius scale of temperature and absolute scale of temperature.
Answer : Let t = Celcius Temperature
T = Kelvin Temperature = t + 273
23 ) What is an ideal gas ?
Answer : A gas that follows gas laws like Boyle’s law, Charles’ Law, Avogadro’s Law etc and an equation PV=nRT, is called as ideal gas. It is only theoritical concept. In reallity, there is no existance of ideal gas. But at a very high temperature and low pressure, the real gas behaves ideally.
24 ) State Charles’ law in terms of absolute temperature.
Answer : It states that the volume of a given mass is directly proportional to its Kelvin temperature while pressure remains constant.
25 ) What is universal gas constant ?
Answer : In equation PV=nRT, ‘R’ is called as universal gas constant. It is independent of nature of the gas, surrounding medium of the gas and other physical quantities like pressure, temperature and volume etc. Its value changes with the system of units used. Its SI vlaue is 8.314 J/mole/K.
26 ) Define gram molecular volume.
Answer : Gram molecular volume is defined as the volume occupied by the one gram mole of a gas at STP.
27 ) What is Avogadro’ s number ?
Answer : Number of particles ( molecules, ions, atom, electrons etc ) in one mole of any substance is called as Avogadro’s Number. Its value is 6.022 * 1024
28 ) What is Avogadro’s law ?
Answer : It states that for a given value of pressure and temperature, equal volume of different gases contains same number of molecules.
Long Answer Type Questions :
29 ) State the characteristics of gases.
Answer : The characteristics of gases aare given below :
i ) Gas hos neither shape nor fixed volume.
ii ) Its volume cahnges with slight change in pressure or temperature.
iii ) Its molecules are of point mass and of point volume.
iv ) There is negligible force of interaction between the gas molecules.
v) The molecules of the gas are always in random motion. It is called as Brownian motion.
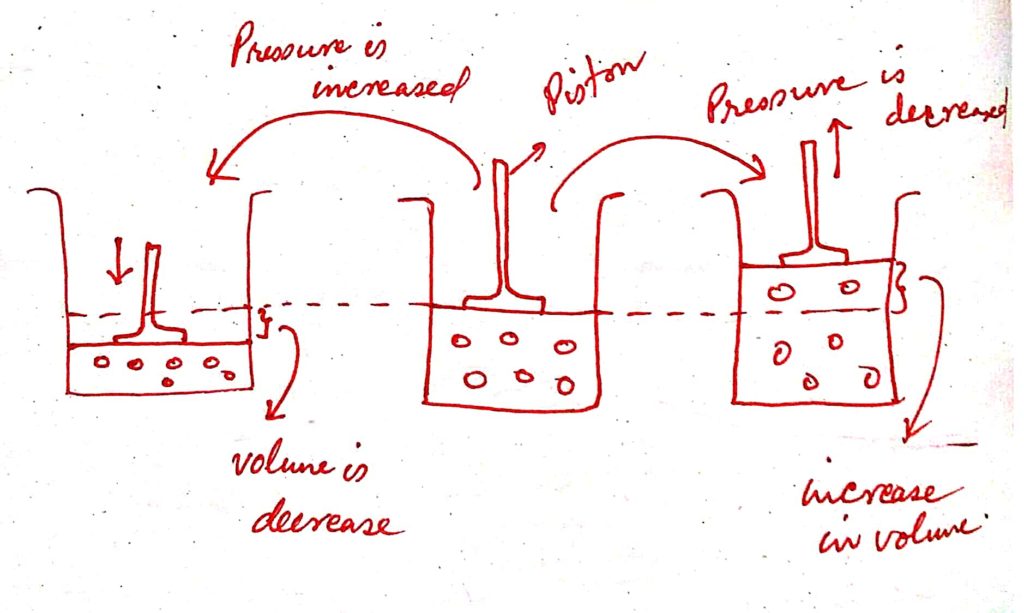
30 ) State Boyle’s law and explain it.
Answer : Statement of Boyles :
It states that the volume of the given mass of a gas is inversly proportional to the pressure of the gas while its temperature temians constant.
Explaination of Boyle’s Law :
Let us assume that a particular gas is enclosed in a closed container. Remember that the nature of the gas remains fixed throughout the experiment. Let us try to keep the temperature of the isolated system constant. After that, the piston is raised in an upward direction. As soon as the piston is raised, there is available more space. As we know that the gas molecules tend to occupy all the available space. In this way, the volume of the gas is increased. Now the piston is moved in a downward direction, all the molecules come to close each other. Hence, the volume of the gas decreased. Thus we have a conclusion, that whenever the pressure increases the volume of the gas decreases and vice – versa but the temperature, mass and nature of the gas should be kept constant.
31 ) State Charles’ law and explain it.
Answer : Statement of Charles’ Law :
It states that the volume of a given mass of a gas is increased or decreased by 1/273rd parts of its initial volume ( or volume at zero degree centigrade ) for every one degree centigrade rise or fall in temperature while pressure remains constant.
Explaination of Charles’ Law :
We know that, if the temperature of the gas increases the average kinetic energy of the gas molecules also increases. The gas molecules tend to occupy more and more space. Hence, the volume will also increase. According to Charles’ Law, if the temperature is increased by one-degree centigrade the volume of the fixed mass of any gas increases 1 / 273rd time of its initial volume but the temperature of the gas remains constant.
32 ) What is absolute zero temperature. Define absolute scale of temperature.
Answer : Absolute zero temperature : A temperature at which the volume of any gas becomes zero, is called as absolute temperature. Its value is -273 0C. This is only theoritical concept. Practically,before reaching this temperature, the state of gas changes into another state like liquid. It is the lowest possible temperature.
Absolute Scale of Temperature : A thermodynamic scale having lower limit as -2730C ( absolute zero temperature ) is called as absolute scale of temperature. It is invented by William Thomson — also known as Lord Kelvin. Therefore, this scale of temperature is also known as Kelvin Scale.
33 ) How is absolute zero obtained from Charles’ laws ?
Answer : At constant pressure, from Charles’ Law, we have
V = V0 ( 1 + t/273 )
where v = volume at absolute zero temperature t0C.
V0 = volume at 00C.
At absolute zero temperature volume becomes zero
V = 0
V0 ( 1 + t/273 ) = 0
or, ( 1 + t / 273 ) = 0
or, t//273 = -1
or, t = -273
Hence, the value of absolute zero temperature is -2730C.
34 ) Establish the combined law of Boyle’s and Charles’ law ? what are the basic assumptions of kinetic theory of gases.
Answer : Combined Form of Boyle’s Law and Charles’ Law :
Let us assume,
P = Pressure of the gas
V = Volume of the gas
T = Kelvin temperature of the gas
M = Mass of the gas
From Boyle’s Law, at constant temperature ( K ) for given mass ( M ) of the gas,
V ∝ 1 / P ————————————- ( 1 )
From Charles’ Law, at constant Kelvin temperatutre and for a given mass of the gas,
V ∝ T ————————————- ( 2 )
By joint variation, we have
V ∝ ( 1 / P ).T
V = K ( T / P )
PV = KT
PV / T = K
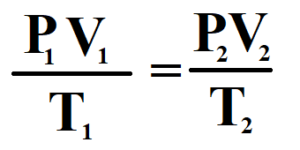
This the combined form of Boyle’s Law and Charles’ Law.
Basic Assumptions of Kinetic Theory of Gas :
i ) Gas molecules are of point mass and of point volume.
ii ) There is negligible force of interaction between the molecules of the gas.
Fill in the blanks :
- Equal volumes of two gases under similar conditions of temperature contain …….. number of molecules.
- The volume of as gas is zero at …. temperature.
- At constant temperature for a given mass of gas the product of its pressure and its volume is ……..
- The velocities of molecules …… with rise of temperature.
- The equation of ideal gas for n gram moles is …
- The value of absolute zero is … on Celsius scale.
Answers of the above blanks : ( 1 ) same ( 2 ) -2730C ( 3 ) constant ( 4 ) increase ( 5 ) PV = nRT ( 6 ) -2730C
State whether true or false :
- All gases known so far are ideal gases.
- No deviation from Avogadro’s law is observed in case of real gases.
- The universal gas constant depends upon the nature of the gas.
- The volume of a gas always decreases if its pressure is decreased.
- The value of Avogadro’s number is 6.022*1023
Answers of above questions : (1) False (2) False (3) False (4) False (5) True.
Match the following :
| Column – I | Column – II |
| 1 ) According to kinetic theory of gases, there are | a ) Obey gas law. |
| 2 In the ideal gs equation PV = nRT, the value of R depends upon | b ) Critical temperature |
| 3 ) The temperature below which a gas does not exist is called | c ) Unit of measurement |
| Avogadro’s number is the number of molecules present in | d ) no inter-molecular attractions |
| 5 ) An ideal gas in one which | e ) 22.4 liters of any gas at STP. |
Answer :
| Column – I | Column – II |
| 1 ) According to kinetic theory of gases, there are | d ) no inter-molecular attractions |
| 2 ) In the ideal gas equation PV = nRT, the value of R depends upon | c ) Unit of measurement |
| 3 ) The temperature below which a gas does not exist is called | b ) Critical temperature |
| 4 ) Avogadro’s number is the number of molecules present in | e ) 22.4 liters of any gas at STP. |
| 5 ) An ideal gas is one which | a ) Obey gas law. |
MCQ- Choose the Correct answer :
- Volume of 4.4 g of CO2 at STP is ——-
- 22.4 L
- 2.24 L
- 224 L
- 44.8 L
- Gas deviates from ideal gas nature because molecules-
- Are colourless
- Attract each other
- Contain covalent bond
- Show Brownian Movement
- The number of gram molecules of oxygen is 6.023 *1024 CO is —
- 8g molecules
- 5g molecules
- 2gm molecules
- 0.5 g molecules.
- A gas will approach ideal behaviour at–
- At low temperature and low pressure
- Low temperature and high pressure
- High temperature and low pressure
- High temperature and high pressure
- Kinetic theory of gases proves–
- Only Boyle’s law
- Only Charles’ law
- Only Avogadro’s law
- All of these.
0 Comments